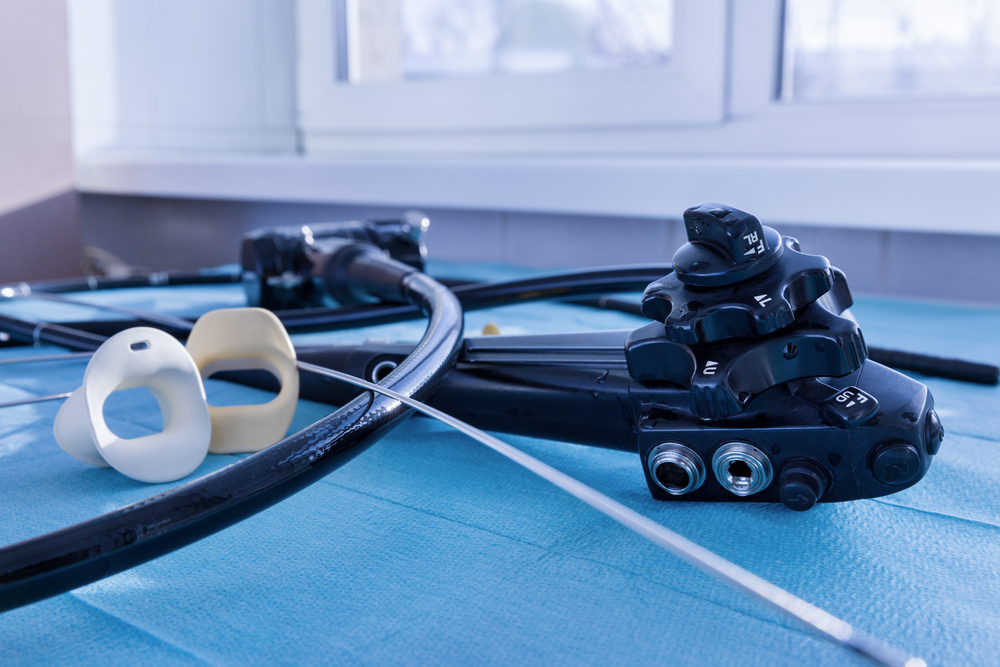
 Individuals looking to decrease their risk for colon cancer by opting for a preventative colonoscopy may be at a risk for exposure to Escherichia coli. A hospital in Washington state reported an outbreak of a rare antibiotic-resistant E. coli strain between November 2012 and August 2013 that is now being linked back to endoscopes in a study published in Infection Control & Hospital Epidemiology, entitled “Endoscopic Retrograde Cholangiopancreatography-Associated AmpC Escherichia coli.”
Individuals looking to decrease their risk for colon cancer by opting for a preventative colonoscopy may be at a risk for exposure to Escherichia coli. A hospital in Washington state reported an outbreak of a rare antibiotic-resistant E. coli strain between November 2012 and August 2013 that is now being linked back to endoscopes in a study published in Infection Control & Hospital Epidemiology, entitled “Endoscopic Retrograde Cholangiopancreatography-Associated AmpC Escherichia coli.”
“Although the endoscopes had been reprocessed according to industry standards, we identified contaminated endoscopes that might have facilitated the transmission of the multidrug-resistant organism,” said Kristen Wendorf, MD, MS, lead author of the study, in a news release. “In the wake of the recent outbreak of carbapenem-resistant E. coli due to contaminated endoscopes, we suspect endoscope-associated transmission of bacteria is more common than recognized and not adequately prevented by current reprocessing guidelines.”
Despite the hospital’s strict adherence to cleaning procedures, 32 patients were infected with carbapenem-resistant E. coli. These patients were not undergoing a colonoscopy. Rather, they had severe pancreatic or biliary disease and required an endoscopic retrograde cholangiopancreatography (ERCP) procedure. More than 30% of the infected patients died during the investigation, with seven of the deaths occurring within 30 days of pinpointing the infecting E. coli isolate. However, it is not possible to determine if infection contributed to deaths, as patients were also affected by colon cancer, pancreatic cancer, primary sclerosing cholangitis, and renal/pancreatic transplants.
The hospital has now taken extraordinary and costly measures to minimize the risk for infection transmitted via endoscope. Already they were adhering to cleaning guidelines, but serious defects in endoscopes not identified during hospital testing allowed bacteria to congregate in the endoscopes’ elevator channel.
“The outbreak was detected through a public health surveillance program that was enhanced with the addition of molecular testing, and would likely have gone undetected otherwise,” said Dr. Wendorf. “Routine surveillance is crucial for promptly recognizing outbreaks and monitoring and responding to the ongoing threat from multidrug-resistant organisms in healthcare facilities.”
Endoscopes are now quarantined after ERCP procedures and will not release them after cleaning and a negative test for culture after 48 hours. Even then, the endoscopes require additional cleaning, as they continue to show signs of bacteria. Reviewing the evaluation and maintenance schedules may be helpful in ensuring adequate cleaning and minimal exposure to bacteria.