 GI View Ltd., an Israel based company committed to develop and advance the efficacy, precision and comfort of colorectal cancer screening, has received a U.S. Food and Drug Administration (FDA) 510(k) clearance for its lead product, the Aer-O-Scope Colonoscope System, which should arrive at the national markets in the beginning of 2016.
GI View Ltd., an Israel based company committed to develop and advance the efficacy, precision and comfort of colorectal cancer screening, has received a U.S. Food and Drug Administration (FDA) 510(k) clearance for its lead product, the Aer-O-Scope Colonoscope System, which should arrive at the national markets in the beginning of 2016.
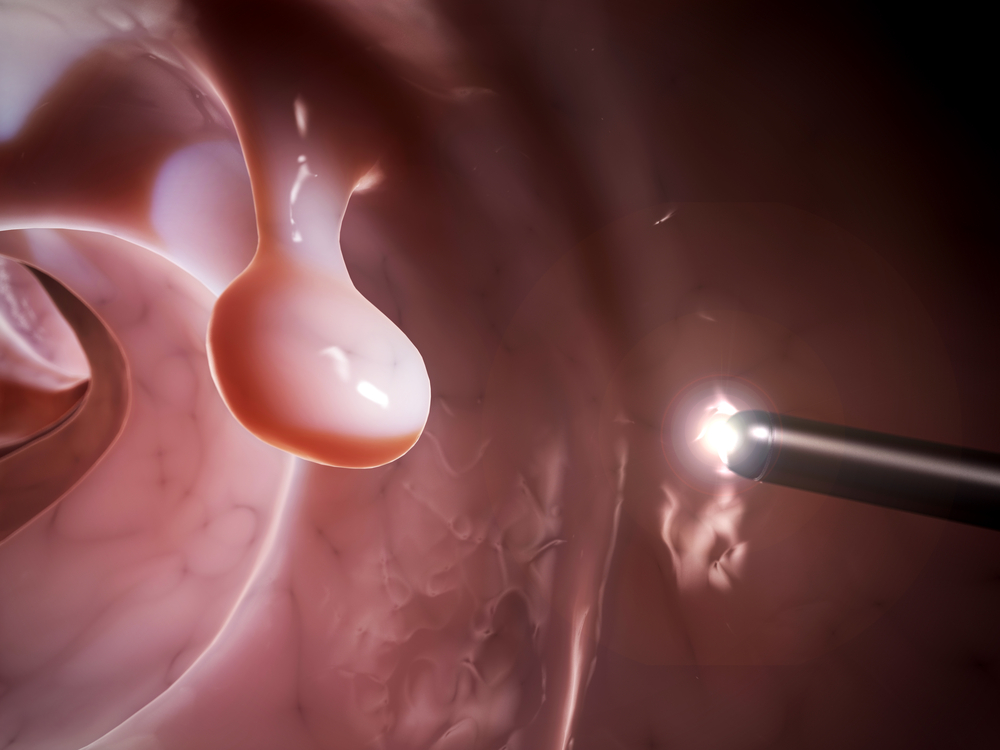
The Aer-O-Scope Colonoscope was developed to provide a safe, easy-to-use solution for GI clinics and hospitals to efficiently screen patients, allowing for an early detection of malignant, pre-cancerous formations, such as polyps.
It possesses a 360° omni-directional visualization, a multi-lumen tube that can decrease pressure on the walls of the colon and increase overall safety. Furthermore, the system is joystick controlled, making it simple to operate.
Importantly, this device is disposable and only allows for a single use, which means it carries no risk of disease transmission.
The FDA clearance was based on promising results from the clinical trial “Clinical Evaluation of the Aer-O-Scope™ Colonoscope System – Pneumatic Assisted Colonoscopy with Panoramic Imaging for Colorectal Cancer Screening”, where researchers found Aer-O-Scope colonoscopy was an easily mastered and safe system that proved equal or superior to conventional colonoscopy in detecting colonic pathologies, an advantage that was especially noticeable in real time detection of lesions of clinically significant dimensions.
“Aer-O-Scope provides reliable and safe colorectal cancer screening. It also has the potential to increase the adenoma detection rate and concurrently help to prevent cancer,” Erwin Santo MD, Head, Department of Gastroenterology and Liver Diseases, Tel Aviv Sourasky Medical Center and principal investigator of the most recent Aer-O-Scope™ clinical trial said in a press release.
“Aer-O-Scope is the only colorectal screening product that is single use, self-propelled and has 360° omni-directional visualization. This enables the physician to observe all the mucosa of the colon, including behind folds, which is critical for a complete colonic assessment. If a polyp is there, Aer-O-Scope™ will allow the physician the best possible chance of finding it,” added Dr. Tal Simchony, CEO of GI-View Ltd.